Our Oncology Registries
Accelerate early development through post-approval with our rich data and global network of leading investigators and sites

Melanoma
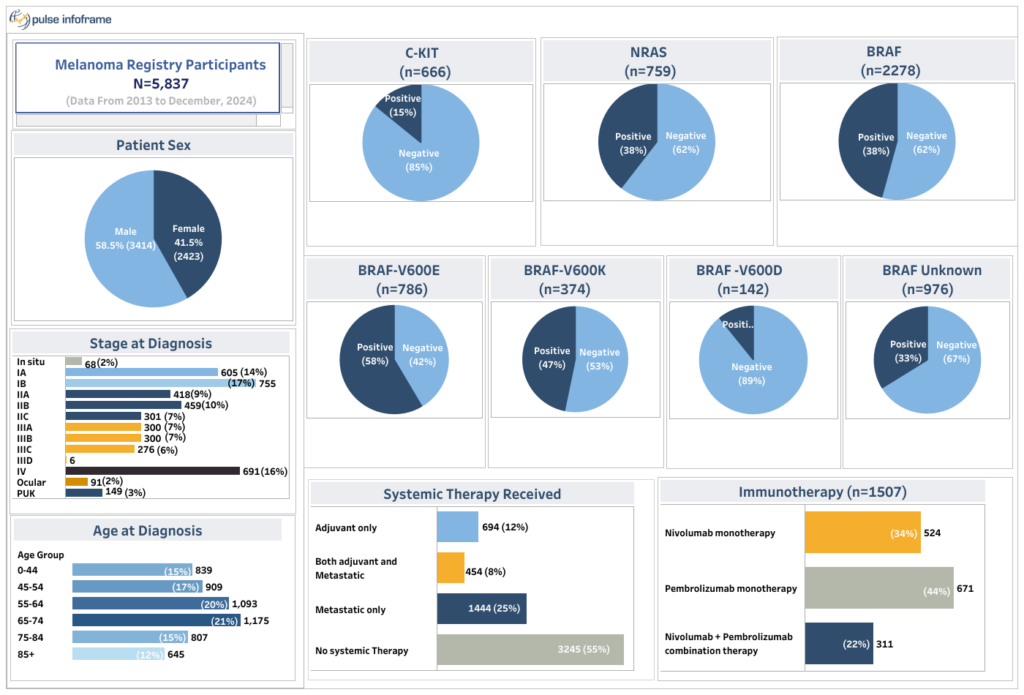 The Global Melanoma Research Network (GMRN), a pioneering initiative established in 2010, has grown into one of the largest global melanoma registries, and now incorporates Merkel Cell Carcinoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma within the overarching skin cancer research network.
The Global Melanoma Research Network (GMRN), a pioneering initiative established in 2010, has grown into one of the largest global melanoma registries, and now incorporates Merkel Cell Carcinoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma within the overarching skin cancer research network.
Data from our Melanoma registry has enabled many of the leading pharma companies to achieve their study objectives through every phase of the drug development lifecycle.
Publications Based on Data from our Melanoma Registry
- Prospective real-world evidence for the use of immune-checkpoint inhibitors and BRAF-targeted therapy in advanced melanoma from a large Canadian cohort. John Gordon Lenehan, D. Scott Ernst, Leah Young, Angel Cronin, Marcus O. Butler, Teresa M. Petrella, Tara D. Baetz, Xinni Song, Tina Cheng, John WT Walker, Linda May Lee, Jen E Melvin, Sudhashree Rajagopal, Femida hussein Gwadry-Sridhar. ASCO 2024 Abstract
- Impact of systemic therapy sequencing on overall survival for patients with advanced BRAF-mutated melanoma. Kartolo BA, Deluce J, Hopman WM, Liu L, Baetz TD, et al. JCO. 2021; 39 )15) suppl_9552.
- Utilization of Real World Data to Assess the Efficacy of Immune Checkpoint Inhibitors (ICI) in Elderly Patients with Metastatic Melanoma. Ernst DS, McConkey H, Gwadry-Sridhar F, Butler M, Baetz T, et al. ESMO 2019, Barcelona, Spain. Poster and oral presentation.
- Impact of Intralesional Interleukin 2 (IL2) for In-transit Melanoma in two Canadian Centers. Ernst DS, Hayward V, McConkey H, Teng X, Saettler E, et al. EMSO 2018, Munich, Germany, Abstract 5477.
- Impact of Ipilmumab on Metastatic Melanoma: Evaluation Using Patient Registry in Canada. Ernst DS, Petrella T, Joshua A, Cheng T, Smylie M, Baetz T, Hamou A, Gwadry-Sridhar F. European Society for Medical Oncology 2016 Congress, Copenhagen, Denmark, October 7-11, 2016. Presented by DS Ernst.
- Burden of illness for metastatic melanoma in Canada, 2011-2013 Ernst, D., Petrella, T., Joshua, A., Hamou, A., Thabane, M., Vantyghem, S., & Gwadry-Sridhar, F. (2016). Current Oncology, 23(6), e563-e570.
- Resource utilization and costs of managing patients with advanced melanoma: a Canadian population-based study. Gwadry-Sridhar F, Nikan S, Hamou A, Seung SJ, Petrella T, Joshua AM, Ernst DS, Mittmann N. (2017). Current Oncology, 24(3), 168-175.
Meet our Principal Investigator
Uveal Melanoma
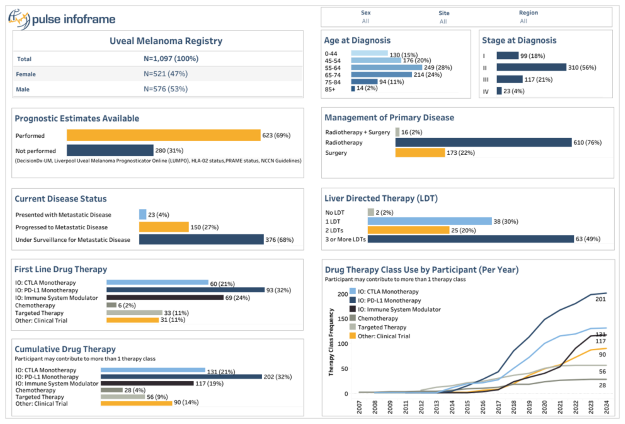 The Ocular Melanoma Natural History Study (OMNi), a groundbreaking initiative that commenced in 2020 and has grown into one of the largest global patient registries for uveal melanoma.
The Ocular Melanoma Natural History Study (OMNi), a groundbreaking initiative that commenced in 2020 and has grown into one of the largest global patient registries for uveal melanoma.
The registry is a collaboration with a global team of primary investigators based in North America, Europe, and Australia. Our collective mission: to establish a dynamic and collaborative network providing unprecedented insights into patients with uveal melanoma on a worldwide scale.
Publications Based on Data from our Uveal Melanoma Registry
Meet our Principal Investigators
Principal Investigator Interviews
Lung Cancer
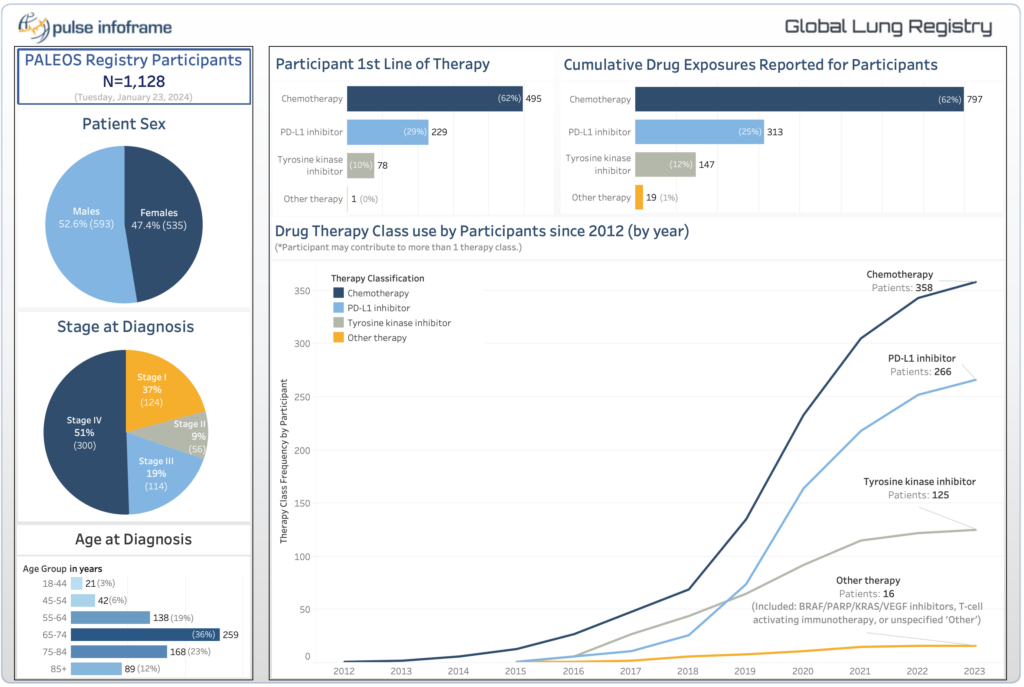 Our Global Lung Cancer Registry was launched in 2020 to shed light on the natural history and real-world treatment patterns of diverse subgroups of lung cancer patients.
Our Global Lung Cancer Registry was launched in 2020 to shed light on the natural history and real-world treatment patterns of diverse subgroups of lung cancer patients.
The registry has grown to 6 sites with plans for continued expansion. Evidence generated from this registry has been published in two manuscripts and multiple abstracts. Our principal investigator, Dr. Parneet K. Cheema was featured in Oncology Treatment Today, describing how real-world evidence from our registries accelerates access to life-saving medications.
Publications Based on Data from our Lung Cancer Registry
Meet Our Principal Investigators
Principal Investigator Interviews
The Pulse Infoframe Difference
For over a decade, Pulse has led critical innovation in the generation of real-world evidence (RWE). Pulse features an advanced technology platform, a global network of investigators and sites, rich data models, large up-and-running longitudinal registries, and deep clinical and regulatory expertise. These assets and approach enable Pulse to better address your research requirements from natural history, through comparator controls, through post-marketing and long-term follow-up.
- Supports critical use cases: Pulse registries support natural history, biomarker and endpoint development, comparator populations, market access, post-marketing, safety and long-term follow-up.
- Up and running longitudinal registries: Leverage an existing registry - The Pulse Platform can expand and enhance to meet your requirements. 35,000+ patients across 24 indications.
- Global site network- fast startup of new registries: Over 150 operational sites and growing across the United States, Canada, Europe, Great Britain, Australia and New Zealand. Can quickly expand within sites to establish new indications as needed.
- Engaged Principal Investigators: Investigators at each site leverage our registry data for their research and publications and are committed to quality.
- Site-based, decentralized, and hybrid models: The Pulse Platform supports all 3 models to enable the right approach to meet your evidence and study objectives.
- Comprehensive data models: Co-designed in partnership with leading investigators and our industry partners to capture elements and data modalities critical to specific indications and contemporary precision medicine.
- Advanced Technology Platform: Cloud-based platform provides customized portals for clinicians, investigators, sponsors and patients. Fully globalized supporting patients in 87 countries and 13 languages.