In 2011, Dr. Femida Gwadry-Sridhar founded Pulse Infoframe to fill a research gap that neglected millions: an inability to collect standardized, useful real world evidence in rare disease, cancer, and chronic condition populations. To increase the usefulness of this data, researchers needed to combine it with more common forms of data, such as medical reports. Collecting such sensitive data also meant developing a platform that would follow stringent regulatory guidelines, that would make it useful to researchers and advocates around the world.
Our Expertise
A History Grounded in Epidemiology
Track Record
10-year history of registries and observational studies in over 25 rare diseases, cancers, and chronic diseases to help thousands navigate their journey toward finding effective treatments and critical answers for their disease or condition.
End-to-End Solutions:
Fully managed, patient-centric solution, including GDPR and HIPAA compliant platform and services that can support multiple types of real-world data (patients, clinicians, EHR, etc.) and meet regulatory submission requirements.
Science-Driven:
Science drives the data. We think long-term to maximize the use of real-world data to accelerate the development of new treatments by considering the scientific research questions our partners want to answer both now and in the future. Guided process led by a dedicated project leader with support from our , clinical team, and data science team as needed to help you determine what data to collect, how, and when.
We Do Data Right:
Quality data collection to maximize use of registry and real-world data
Real-World Evidence
Our Platform approach is designed to support the collection of real-world data that is structured to ensure research methods can be systematically applied to produce meaningfully comparable and reproducible evidence.
This approach enables you to address your research questions in two ways:
- by accessing existing data sets from ongoing registries through a transparent governance structure, or
- by taking a targeted approach through the designment of a framework and platform that enables the collection of real-world data to address your specific research questions.
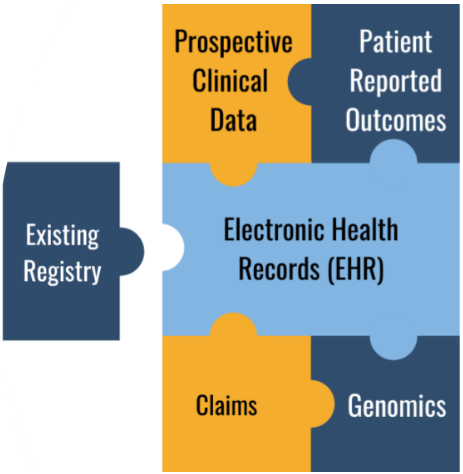
Multiple Data Types
Our team of clinical and data science experts will work with you to home in on the data types and variables you need to address your study objectives and research questions and minimize the collection of unnecessary data that will only generate noise. Once we understand your data requirements, our platform supports the collection of data sources such as Existing registry data, EHR, claims, PROs, genomics, and more.
Standardized Data
Our Platform enables the structured collection of data and can map it to Observational Medical Outcomes Partnership (OMOP), Clinical Data Interchange Standards Consortium (CDISC), and others to meet downstream pharma regulatory requirements. We ensure the data captured allows for consented data-sharing permissioning across stakeholders and meets regulatory standards required by the FDA and EMA.
Secure Data
Our Platform is GDPR and HIPAA-compliant.